Hematopoiesis
 Hematopoiesis is the formation of blood cellular components, which are derived from haematopoietic stem cells. In a healthy adult person, approximately one thousand new blood cells are produced daily in order to maintain steady-state levels in the peripheral circulation.
Hematopoiesis is the formation of blood cellular components, which are derived from haematopoietic stem cells. In a healthy adult person, approximately one thousand new blood cells are produced daily in order to maintain steady-state levels in the peripheral circulation.
Leukemia
 Leukemia is cancer of blood or bone marrow cells. These blood cells form in healthy bone marrow. In leukemia, however, the bone marrow produces abnormal blood cells. These cells crowd out the healthy blood cells, making it difficult for the blood to function properly. There are different types of leukemia, including Acute lymphocytic leukemia (ALL), Acute myeloid leukemia (AML); Chronic lymphocytic leukemia (CLL); and Chronic myeloid leukemia (CML) Unlike cancers of solid organs, leukemia cells circulate throughout the immune system and therefore cannot be specifically targeted to be eliminated. The only available current treatments for leukemia are systemic, such as chemotherapy or radiation therapy.
Leukemia is cancer of blood or bone marrow cells. These blood cells form in healthy bone marrow. In leukemia, however, the bone marrow produces abnormal blood cells. These cells crowd out the healthy blood cells, making it difficult for the blood to function properly. There are different types of leukemia, including Acute lymphocytic leukemia (ALL), Acute myeloid leukemia (AML); Chronic lymphocytic leukemia (CLL); and Chronic myeloid leukemia (CML) Unlike cancers of solid organs, leukemia cells circulate throughout the immune system and therefore cannot be specifically targeted to be eliminated. The only available current treatments for leukemia are systemic, such as chemotherapy or radiation therapy.
Hematopoietic stem cell transplantation (HSCT)
 As is all cancers including leukemia, the difficulty of eliminating cancer cells is their similarity to normal cells as they begin as and look almost like normal cells. The major difference is their speed of growth – cancer cells are particularly fast growing but the characteristic of some normal cells such as hair and blood cells are also subject to fast growth. The mechanism of action of current anti-cancer drugs is to selectively interfere with cell growth and/or function of fast growing cells. However, a common side effect of these treatments causes damage to other fast growing cells. Most significant is the effect on hematopoietic stem cells (HSCs) that continuously generate red blood cells (RBCs), white blood cells (WBCs) and platelets. To reduce the damage to HSCs, HSCT from a human donor is therefore essential to reconstitute a normal blood producing system.
As is all cancers including leukemia, the difficulty of eliminating cancer cells is their similarity to normal cells as they begin as and look almost like normal cells. The major difference is their speed of growth – cancer cells are particularly fast growing but the characteristic of some normal cells such as hair and blood cells are also subject to fast growth. The mechanism of action of current anti-cancer drugs is to selectively interfere with cell growth and/or function of fast growing cells. However, a common side effect of these treatments causes damage to other fast growing cells. Most significant is the effect on hematopoietic stem cells (HSCs) that continuously generate red blood cells (RBCs), white blood cells (WBCs) and platelets. To reduce the damage to HSCs, HSCT from a human donor is therefore essential to reconstitute a normal blood producing system.
Human leukocyte antigens (HLAs)
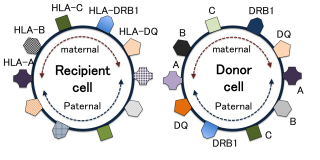 HLAs are proteins — or markers — found on most cells in the body. The immune system uses these markers to recognize which cells belong (normal) and which do not (foreign). In HSCT, a match of at least 6 of these 8 (or 8 out of 10) HLA markers is required for adult donors. Such matched donors are difficult to find, although they are found more often in siblings. Bone-marrow banks from anonymous donors offer the only alternative, although the success rate in finding a match is quite low.
HLAs are proteins — or markers — found on most cells in the body. The immune system uses these markers to recognize which cells belong (normal) and which do not (foreign). In HSCT, a match of at least 6 of these 8 (or 8 out of 10) HLA markers is required for adult donors. Such matched donors are difficult to find, although they are found more often in siblings. Bone-marrow banks from anonymous donors offer the only alternative, although the success rate in finding a match is quite low.
Graft-versus-Host Disease (GvHD)
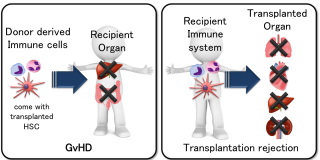 GvHD is a serious complication that results from rejection of the donor HSCT. GvHD is caused by the donor’s immune system recognizing the cells/organs derived from the patient (recipient) as pathogens (foreign body). This can occur even with an HLA-matched donor’s HSC; GvHD actually occurs in most of the patients, regardless of the donor. A low grade of GvHD is regarded as positive, because it reflects a normal immune reaction while trying to eliminate residual cancer cells. However, a more severe grade of GvHD can be life threatening as it may significantly damage skin cells, fast growing cells in the gastrointestinal (GI) tract and liver cells.
GvHD is a serious complication that results from rejection of the donor HSCT. GvHD is caused by the donor’s immune system recognizing the cells/organs derived from the patient (recipient) as pathogens (foreign body). This can occur even with an HLA-matched donor’s HSC; GvHD actually occurs in most of the patients, regardless of the donor. A low grade of GvHD is regarded as positive, because it reflects a normal immune reaction while trying to eliminate residual cancer cells. However, a more severe grade of GvHD can be life threatening as it may significantly damage skin cells, fast growing cells in the gastrointestinal (GI) tract and liver cells.
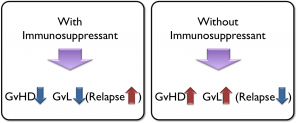 The current option to treat GvHD is to use immune suppressant drugs, which work well in terms of treating the GvHD. However, these drugs are designed to work by shutting down all immune reactions. As a result, they increase the risk of infection and relapse, which are major causes of death following HSCT. Therefore, only choices for patients are (1) to use immunosuppressants to reduce GvHD but increase risk of infection and relapse OR (2) to choose no treatment to avoid relapse, but suffer from the consequences of GvHD.
The current option to treat GvHD is to use immune suppressant drugs, which work well in terms of treating the GvHD. However, these drugs are designed to work by shutting down all immune reactions. As a result, they increase the risk of infection and relapse, which are major causes of death following HSCT. Therefore, only choices for patients are (1) to use immunosuppressants to reduce GvHD but increase risk of infection and relapse OR (2) to choose no treatment to avoid relapse, but suffer from the consequences of GvHD.
RGI-2001
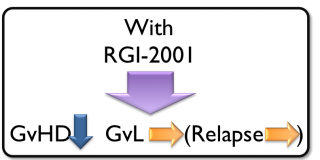 RGI-2001 is a liposomal formulation of alpha-GalCer (a CD1d ligand), which induces regulatory T cells (Treg), a key player in immune tolerance. Treg has been shown to have significant potential for treating Graft-versus-Host Disease (GvHD). In studies by independent researchers, Treg has proven to provide longer patient survival because it reduces rejection without reducing a graft-versus-leukemia/tumor (GvL) effect. Unlike immunosuppressents that destroy entire T cell subsets, RGI-2001 induces Treg while maintaining normal immune cell functions and has the potential to reduce GvHD and improve survival for patients. The company has received orphan drug designation from the US FDA for developing this compound for the prevention of GvHD associated with hematopoietic stem cell transplantation.
RGI-2001 is a liposomal formulation of alpha-GalCer (a CD1d ligand), which induces regulatory T cells (Treg), a key player in immune tolerance. Treg has been shown to have significant potential for treating Graft-versus-Host Disease (GvHD). In studies by independent researchers, Treg has proven to provide longer patient survival because it reduces rejection without reducing a graft-versus-leukemia/tumor (GvL) effect. Unlike immunosuppressents that destroy entire T cell subsets, RGI-2001 induces Treg while maintaining normal immune cell functions and has the potential to reduce GvHD and improve survival for patients. The company has received orphan drug designation from the US FDA for developing this compound for the prevention of GvHD associated with hematopoietic stem cell transplantation.
Clinical trial

A Phase II study of multiple doses of RGI-2001 for GvHD associated with hematopoietic stem cell transplantation has been initiated, The safety and efficacy clinical trial will enroll approximately 50 bone marrow or peripheral blood stem cell transplantation leukemia patients following chemotherapy. The U.S.-based study is being conducted in eight leading cancer centers. Clinical centers are currently recruiting patients. For information regarding participation in the Phase II study of RGI-2001 for GvHD please visit https;
A Phase I first in human of RGI-2001 dose escalation was completed. The manuscript is published, Chen et al, 2017 ClinicalTrials.gov